- Home
- Publications
- GIGA Focus
- Antimicrobial Resistance between Lack of Access and Excess
GIGA Focus Global
Antimicrobial Resistance between Lack of Access and Excess
Number 3 | 2020 | ISSN: 1862-3581
The use of antimicrobials, primarily antibiotics, but also antivirals, antiparasitics, and antiseptics is rapidly rising around the world – and with it, antimicrobial resistance (AMR). The current COVID-19 pandemic illustrates both the burden on the health system and the economic costs a pathogen can pose when no medication is available. Concerning resistance, the crux of the matter lies in balancing the need to reduce overall antibiotic consumption while at the same time expanding access in countries where such antibiotics are not easily accessible.
The rising use of antibiotics in health and agricultural sectors are driving the increase in antimicrobial resistance. The quick expansion of access and use along with ineffective stewardship have led to alarming resistance rates, particularly in fast-growing middle-income economies – a problem these countries must urgently address.
If AMR is not tackled more efficiently, it is estimated that by 2050 ten million people will die each year from common infections that will then no longer be treatable. Concomitant with this will be substantial economic losses predicted to total 2.5 to 3 per cent of global GDP. Hence, the pressure is mounting to secure more international commitment to combat AMR.
Lowering antibiotic consumption might slow the pace of the spread of resistance, as every dose raises the risk of resistance building. Hence, the continuous development of new medicines is crucial and requires market intervention to take place.
An oft-neglected aspect of the debate over AMR is that especially in low- and middle-income countries many life-saving antibiotics are not or not sufficiently available. This situation undermines controlled distribution and promotes a vicious cycle of poverty and antimicrobial resistance.
Policy Implications
Antimicrobial resistance is a serious concern and, particularly given the presence of COVID-19, one where action should not be delayed. Policies need to address three issues: First, emerging economies must implement effective policy measures to combat resistance. Second, public funding is required to remedy market failure. Third, international cooperation, particularly in research on diagnostics and prevalence as well as interventions, is necessary to better assess the societal impact of antimicrobial resistance and identify efficient ways to actively address the problem.
Antimicrobial resistance (AMR) occurs when microorganisms such as bacteria, viruses, fungi, and parasites mutate such that common medicines are no longer effective to treat the infections they cause. Because antibiotics, used to treat bacterial infections, are in wider use than antiviral drugs, we focus on antibiotic resistance in the following. Antibiotic resistance is a subcategory of AMR referring to bacteria resistant to antibiotics. These so-called “superbugs” are classified as multi-drug-resistant (MDR) pathogens when non-susceptible to at least one agent in three or more antimicrobial categories, extensively drug-resistant (XDR) if bacterial isolates remain susceptible to only one or two categories, and pan-drug-resistant (PDR) if they are non-susceptible to all agents in all antimicrobial categories. The more resistances, the more difficult such microorganisms are to treat and control and the bigger threat they pose.
The Use of Antibiotics Is Rising
Antibiotics are indispensable for human health. They are essential for treating bacterial infections and performing surgical interventions, and their use is increasing around the world. Between 2000 and 2015, the global consumption of antibiotics measured in terms of defined daily doses (DDD) increased by 65 per cent (Klein et al., 2018). This increase is largely driven by a rise in consumption in emerging economies. In India, at the top of the list, antibiotic consumption more than doubled in that 15-year period (Klein et al., 2018).
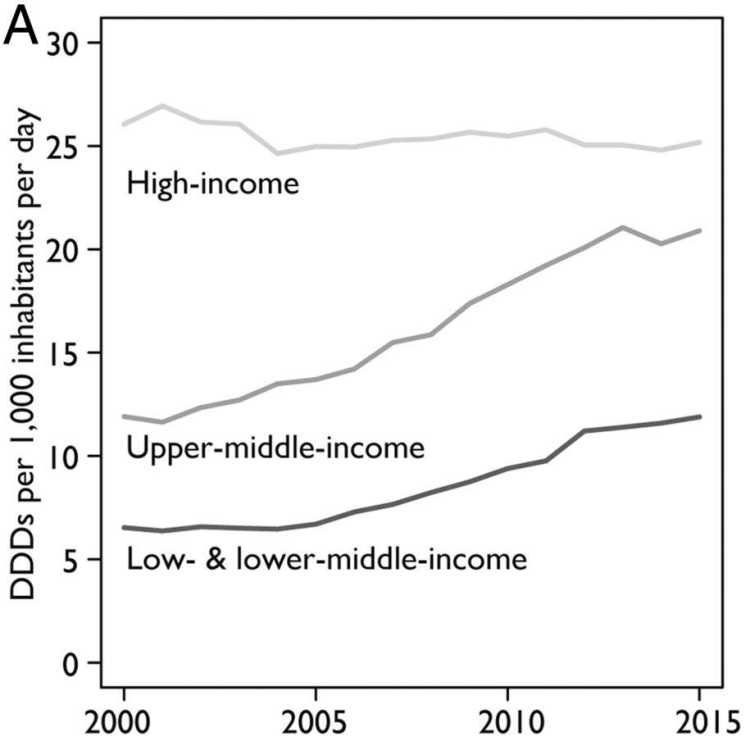
Antibiotics are used not only for human but also for animal health. Moreover, they also find application in food-animal production: as growth hormones and for sanitation purposes. Similar to the developments in human health, the consumption of antibiotics is also increasing in agriculture. By 2030 the consumption of antibiotics in food-animal production is projected to rise by 67 per cent globally. Again, the driver of this escalation is an increasing consumption in fast-growing middle-income economies, especially Brazil, Russia, India, China, and South Africa. Given both expected growth in the demand for livestock products and the shift to large-scale farming in these countries, the use of antibiotic growth promoters (AGPs) is expected to double by 2030 (Van Boeckel et al. 2015). Experts believe that the global consumption of antibiotics in animals is twice that of humans (Aarestrup, 2012).
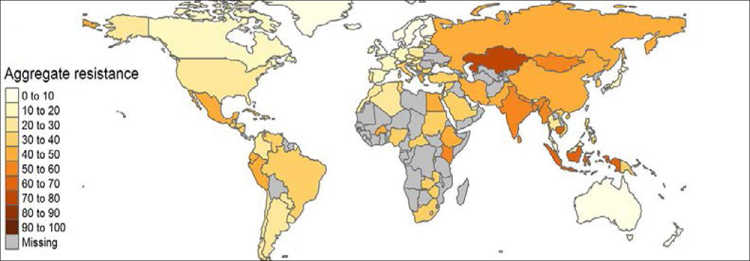
Current trends and projections point to a shift in antibiotic consumption from high-income countries to emerging economies (see Figure 1). This is consistent with the resistance rates we observe. In some countries in the Global South, particularly in Asia, resistance rates are reaching alarming levels (see Figure 2). In response to the growing resistance levels, the World Health Organization (WHO) has classified 13 pathogens at risk of becoming multi-resistant. The most pressing concern with respect to these bacteria is the reduced effectiveness of so-called “last-line” antibiotics. Although these constitute the last alternative medicines, worrying resistance rates have been measured for some of them around the globe. In India and Nigeria, for example, resistance levels for third-generation cephalosporins and carbapenems – two of these last-line antibiotics – are as high as 80 per cent.
The Costs Are Considerable
Each year, 700,000 people die due to AMR, including antimalarial and antiviral resistance (Review on Antimicrobial Resistance 2016). This compares to 17.8 million deaths due to cardiovascular diseases – the leading cause of death worldwide. If the current trends continue, however, deaths due to AMR could become a close second. By 2050 the AMR death toll is expected to reach ten million a year (Review on Antimicrobial Resistance 2016). The largest burden will be in the Global South: of all AMR-related deaths, 90 per cent are expected to occur in Africa and Asia (Review on Antimicrobial Resistance 2016).
In addition to the loss of human lives, the economic costs will also be substantial. The estimated magnitudes are in the range of 2.5 to 3 per cent of the world’s GDP. This is equivalent to a loss of USD 100 trillion by 2050 (Review on Antimicrobial Resistance 2016). These estimates are similar to the costs associated with a 2°C rise in global average surface temperature (Roope et al. 2019). Yet, these numbers have to be taken with a pinch of salt. The economic costs of AMR are fraught with uncertainty and a lack of reliable data, maybe even more so than the estimates for climate change. For many countries, reliable information on resistance levels is lacking (see Figure 1); for those countries in the Global South for which we do have data, rates are often based on lower-frequency, highly selected samples and thus estimated with a large margin of error.
Fighting AMR
Tackling AMR requires ending overuse of antibiotics. But what is the alternative? High-income countries use a system of controlled distribution, with physicians prescribing and pharmacies selling antibiotics. In emerging and even more so in low- and middle-income countries (LMIC), consumption is greatly influenced by unregulated supply chains and by purchase without prescriptions over the counter (OTC). A systematic review by Sakeena et al. (2018) shows that in LMICs 60 per cent of antibiotics are sold OTC without prescription. The resulting self-medicating represents a major problem in LMICs, one that has been largely neglected. While making prescriptions mandatory may solve the problem, it might not be enough. In high-income countries, where OTC sales are more regulated, rates of erroneous or inadequate prescriptions by doctors are extremely high. Recent estimations indicate that approximately one-third of antibiotic prescriptions in the United States were unnecessary. For the United Kingdom, the numbers are even higher: experts found that over 80 per cent of the prescriptions there were unnecessary (Fleming-Dutra et al. 2016; Pouwels et al. 2018).
What we need is a more in-depth understanding of how antibiotics are prescribed and consumed. Sensitising and incentivising health professionals and the public vis-à-vis when and how to use antibiotics (e.g. not to self-medicate, and to adhere to the treatment schedule) could discourage improper use. Yet, thus far, we have little systematic evidence on how overcoming information constraints actually affects antibiotic use. From other information interventions on sensitising health professionals or patients to drug use, treatment, and preventive measures in health, which have mostly focused on the short term, we can only conclude that effects are mixed and very context-specific.
Over-prescription could also be reduced by access to better diagnostics. This issue is particularly relevant in resource-poor settings, which lack clinical diagnostics for accurate treatment. In contexts with a high burden of diseases, health practitioners often prescribe more aggressive antibiotics than are actually needed, to be on the “safe” side. In Malawi, for example, health practitioners are encouraged to treat all patients with fever with antibiotics or write prescriptions based on availability rather than suitability – practices that inevitably result in over-prescription.
In consequence, reducing antibiotic use to socially desirable levels will require better tools and information, a better understanding of the incentives of consumption, and better monitoring and stewardship. This applies not only to health but also to agriculture. Stricter regulation of AGPs is a step in the right direction. The European Union’s tightened restrictions on prophylactic antibiotics have proven fruitful; to take one example, the quantity of veterinary antibiotics sold in Germany dropped 57 per cent between 2011 and 2017 (German Federal Ministry of Health 2019). By contrast, Brazil is the only emerging economy with at least a partial ban on AGPs.
Reductions in the use of antibiotics, however, will only slow the pace at which resistances generate: susceptibility to current antibiotics will continue to decrease. Hence, it is vital to develop new, effective antibiotics. Of course, such development is costly, and if left to the market, it is unlikely that we will see timely progress. Most of the major pharmaceutical companies – 21 of the top 25 companies – have stopped research and development (R&D) on antibiotics. Since antibiotics should be used scarcely in order to keep resistances at bay, the development of new antibiotics is not lucrative – a classic case of market failure.
Research on new antibiotics is now mostly in the hands of start-ups and small and medium enterprises (SMEs), which lack the financial capacity to push their products through the evaluation processes necessary for market authorisation. The most challenging part – called “the valley of death” – is to pass from the preclinical (animal testing) to the clinical (human testing) development stage. After that, the financial hurdle is still not easy to overcome: 80 per cent of the costs for new antibiotics are spent on the operational costs of moving an antibiotic from phase one to phase three of clinical trials, an amount estimated to be upwards of USD 130 million (Review on Antimicrobial Resistance 2016). Taking into account that only 14 per cent of drugs enrolled in clinical development reach the market, the risk for developers is high (Wong et al. 2019).
To date there are 138 antibiotics-related projects in the pipeline (Antimicrobial Resistance Access to Medicines Foundation Report 2020). That might sound like a lot, but to put it into perspective there are more than 800 projects in oncology. Furthermore, since 1990, no novel classes of antibiotics with distinct chemical structures have reached the market. In light of the reduced research activities and the high financial costs, if and when new antibiotics reach the market remains an open question.
The market alone will thus not generate a sufficient number of new antibiotics, so innovative approaches must be taken to overcome the market failure and incentivise R&D in new antibiotics. Three options are plausible:
Using public funds to financially reward development, similar to the approach successfully used by the Drugs for Neglected Diseases initiative (DNDi). Reward-based incentives financed by a global fund would not only increase the quantity of R&D projects but also allow the direction of research to be determined by something other than sales figures. This approach has also been proven effective by the Global Fund to Fight AIDS, Tuberculosis and Malaria. Rewards would need to constitute about USD 1.6 billion per year to effectively “de-link” profitability from sales volume. This is about three times the amount currently spent by the public sector worldwide to promote AMR-relevant research. Hence, more resources would need to be redirected – something unlikely to happen in the short run.
Extending patent protections. For example, the US Food and Drug Administration (FDA) confers market exclusivity for the development of drugs targeting rare diseases, during which time no generics can be approved.
Establishing a “fast-track” approval process combined with a tradeable voucher, similar to the one created by the FDA to tackle tropical and rare paediatric diseases. Such a programme would provide companies a tradeable voucher that allows the corporation to have any one of their drugs evaluated more quickly.
The Real Problem Might Be Access Not Excess
While there is increasing recognition that antimicrobial resistance is a serious public health problem, one important issue has been overlooked. Currently, more people die from a lack of access to antibiotics than from resistant bacterial pathogens. The estimated 700,000 deaths from antibiotic-resistant infections are substantially outnumbered by the 5.7 million deaths each year – most occurring in LMICs – that could be prevented by antibiotics.
Thus far, the lack of access to antibiotics has received comparably little attention. Reasons for lack of access are poorly understood, and potential solutions have gone largely unexplored. The problem is often reduced to one of financial constraints. Despite an increasing push for universal health insurance in LMICs, out-of-pocket expenditures for health are still high, with households spending between 25 and 70 per cent of their income on health care and medicines. Because of the high costs, patients often buy incomplete courses, which in turn could both lead to treatment failure and promote resistance, creating a vicious cycle. As bacteria are becoming resistant to first-line antibiotics, there is a need for second- and third-line antibiotics, which are more expensive. In some countries, second- and third-line antibiotics can cost up to 60 times the price of first-line medicines, even when all of the latter are generic. Higher costs for the treatment of resistant bacteria are putting people at risk of slipping into poverty. Studies estimate that 28.3 million people could fall into extreme poverty by 2030 because of antimicrobial resistance (Ahmed et al. 2018). Yet, we have insufficient empirical evidence on which groups are most affected by the lack of access, how access varies by income level, or the implications of these patterns. This is an important factor to consider when discussing policy interventions (subsidies being just one example) to overcome the access problem.
The access problem, however, has several layers. It is not only one of not being able to afford medicines, but also one of poor-quality medicines circulating in the market. Of the substandard or falsified medicines reported to the WHO, 17 per cent are antibiotics. Substandard antibiotics cause 169,000 childhood pneumonia deaths each year. Yet, the problem goes even further. Similar to pursuing R&D for new antibiotics, producing antibiotics is also becoming less and less interesting for pharmaceutical companies. This has implications for entire supply chains and will aggravate the provisioning of antibiotics in LMICs. Currently, manufacturers already supply only 14 out of 30 established antibiotics to LMICs (Antimicrobial Resistance Acces to Medicines Foundation Report, 2020). For novel antibiotics, the picture is even bleaker: according to the 2020 Antimicrobial Resistance Benchmark Report, only three of the 13 patented novel antibiotics are available in more than ten of the LMICs.
Walking the Line: Expanding Access without Generating Excess
It is evident that we must reduce excess use of antibiotics. Yet, it is also critical to remember that in low- and middle-income countries the still bigger problem is one of access rather than overconsumption. Increasing access to antibiotics is essential to reducing the infectious disease burden in LMICs. However, expanding access without stewardship and regulation is dangerous. The fast-growing middle-income countries are a case in point. Without sufficient controls, resistance rates can quickly rise to alarming rates, as we have seen in India and Nigeria.
Diseases and bacteria travel beyond traditional borders – the current COVID-19 pandemic illustrates that pertinently. The COVID-19 pandemic has also shown that in times of crisis, countries resort to national measures, even though a coordinated, international approach might be more efficient in controlling and overcoming the crisis; in light of the steep initial development of the crisis, a coordinated response seemed out of reach. Though, like COVID-19, AMR does not respect borders, it is nevertheless a predictable threat. Resistances spread as fast as their carriers do. Whether viruses, bacteria, or other microorganisms, they all use waterways, food chains, or humans to jump onto their next host. Intertwined travelling networks and international supply chains make it easy for pathogens to travel across the globe. To date [27.03.2020], USD 7 trillion has been spent worldwide to stop the spread of COVID-19 (Horowitz 2020). This shows the potential of governments to respond with vast sums of money in a short period of time to counteract an acute global threat. Antibiotic resistance is not yet classified as an acute crisis for which short-term measures need to be taken. Rather, we are at a point where structural and longer-term actions should be taken to address the threat of antimicrobial resistance more cost-effectively and sustainably.
Improving access needs to be at the fore. Addressing infections treatable with antibiotics “at home” will eventually help prevent the rise of superbugs. If resistance renders treatments ineffective, efforts to improve access to antibiotics will be futile, with global consequences. Antibiotic stewardship and infection prevention must, therefore, be pursued alongside improvements in access. Balancing access and excess requires the engagement of all players in the health system along with holistic interventions to educate consumers, incentivise the pharmaceutical sector, and promote access to effective antibiotics globally. Small efforts are being made in this direction. The Global Antibiotic Research and Development Partnership (GARDP) – an international non-profit organisation – is working to ensure sustainable access to newly produced antibiotics. More recently, the FDA, the European Medicines Agency, and the Japanese Pharmaceuticals and Medical Devices Agency joined together to reduce the requirements for clinical data on drugs for infections with limited treatment options to speed up the time to market. However, the focus should be on low- and middle-income countries, which receive vaccines and medicines four to seven years after high-income countries have access to them (Ahonkhai et al. 2016). Supply chains are still insufficiently expanded, and inconsistent regulatory standards represent real barriers in LMICs. Organisations such as the African Medicines Regulatory Harmonization (AMRH) initiative, which attempts to improve medicine registration in Africa through cost-effective use of the limited financial, technical, and human resources available, are a step in the right direction.
Footnotes
References
Aarestrup, F. (2012), Sustainable Farming: Get Pigs off Antibiotics, in: Nature, 486, 465–466, www.nature.com/articles/486465a (02 April 2020).
Ahmed, S. A., Bariş, E., Go, D. S., Lofgren, H., Osorio-Rodarte, I., and Thierfelder, K. (2018), Assessing the Global Economic and Poverty Effects of Antimicrobial Resistance, in: World Development, 111, 148-160, https://ideas.repec.org/a/eee/wdevel/v111y2018icp148-160.html (02 April 2020).
Ahonkhai, V., Martins, S. F., Portet, A., Lumpkin, M., and Hartman, D. (2016), Speeding Access to Vaccines and Medicines in Low- and Middle-Income Countries: A Case for Change and a Framework for Optimized Product Market Authorization, in: PLOS ONE, 11, 11, https://doi.org/10.1371/journal.pone.0166515 (02 April 2020).
Antimicrobial Resistance Acces to Medicines Foundation Report (2020), Retrieved from: www.amrbenchmark.org (02 April 2020
Fleming-Dutra, K. E., Hersh, A. L., Shapi).ro, D. J., Bartoces, M., Enns, E. A., File, T. M., and Hicks, L. A. (2016), Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010-2011, in: JAMA - Journal of the American Medical Association, 315, 17, 1864–1873, https://doi.org/10.1001/jama.2016.4151 (02 April 2020).
German Federal Ministry of Health (2019), DART 2020, 4th Interim report 2019, www.bundesgesundheitsministerium.de/fileadmin/Dateien/5_Publikationen/Praevention/Broschueren/DART2020_4-Zwischenbericht_2019_EN.pdf (02 April 2020).
Heem Wong, C., Wei Siah, K., and Lo, A. W. (2019), Estimation of Clinical Trial Success Rates and Related Parameters, in: Biostatistics, 20, 273–286, https://doi.org/10.1093/biostatistics/kxx069 (02 April 2020).
Horowitz, J. (2020), The Bill for Saving the World Economy is $7 Trillion and Rising, CNN Business, https://edition.cnn.com/2020/03/26/economy/global-economy-coronavirus-bailout/index.html (31 March 2020).
Klein, E. Y., Van Boeckel, T. P., Martinez, E. M., Pant, S., Gandra, S., Levin, S. A., and Laxminarayan, R. (2018), Global Increase and Geographic Convergence in Antibiotic Consumption between 2000 and 2015, in: Proceedings of the National Academy of Sciences of the United States of America, 115, 15, E3463–E3470, https://www.pnas.org/content/115/15/E3463 (02 April 2020).
Pouwels, K. B., Christiaan Dolk, F. K., M Smith, D. R., Robotham, J. V, and Smieszek, T. (2018), Actual Versus “Ideal” Antibiotic Prescribing for Common Conditions in English Primary Care, in: Journal of Antimicrobial Chemotherapy, 73, 2, https://doi.org/10.1093/jac/dkx502 (02 April 2020).
Roope, L. S. J., Smith, R. D., Pouwels, K. B., Buchanan, J., Abel, L., Eibich, P., and Andordsworth, S. (2019), The Challenge of Antimicrobial Resistance: What Economics Can Contribute, in: Science, 364, 6435, https://doi.org/10.1126/science.aau4679 (02 April 2020).
Sakeena, M. H. F., Bennett, A. A., and McLachlan, A. J. (2018), Non-prescription Sales of Antimicrobial Agents at Community Pharmacies in Developing Countries: A Systematic Review, in: International Journal of Antimicrobial Agents, 52, 771–782, https://doi.org/10.1126/science.aau4679 (02 April 2020). T
Tackling Drug-Resistant Infection Globally: Final Report And Recommendation (2016), The Review on Antimicrobial Resistance, https://wellcomecollection.org/works/thvwsuba (02 April 2020).
Van Boeckel, T. P., Brower, C., Gilbert, M., Grenfell, B. T., Levin, S. A., Robinson, T. P., and Laxminarayan, R. (2015), Global Trends in Antimicrobial Use in Food Animals, in: Proceedings of the National Academy of Sciences of the United States of America, 112, 18, 5649–5654, https://doi.org/10.1073/pnas.1503141112 (02 April 2020).
General Editor GIGA Focus
Editor GIGA Focus Global
Editorial Department GIGA Focus Global
Regional Institutes
Research Programmes
How to cite this article
Hartwig, Renate, and Pascal Segura Kliesow (2020), Antimicrobial Resistance between Lack of Access and Excess, GIGA Focus Global, 3, Hamburg: German Institute for Global and Area Studies (GIGA), https://nbn-resolving.org/urn:nbn:de:0168-ssoar-67333-2
Imprint
The GIGA Focus is an Open Access publication and can be read on the Internet and downloaded free of charge at www.giga-hamburg.de/en/publications/giga-focus. According to the conditions of the Creative-Commons license Attribution-No Derivative Works 3.0, this publication may be freely duplicated, circulated, and made accessible to the public. The particular conditions include the correct indication of the initial publication as GIGA Focus and no changes in or abbreviation of texts.
The German Institute for Global and Area Studies (GIGA) – Leibniz-Institut für Globale und Regionale Studien in Hamburg publishes the Focus series on Africa, Asia, Latin America, the Middle East and global issues. The GIGA Focus is edited and published by the GIGA. The views and opinions expressed are solely those of the authors and do not necessarily reflect those of the institute. Authors alone are responsible for the content of their articles. GIGA and the authors cannot be held liable for any errors and omissions, or for any consequences arising from the use of the information provided.


















